TOP>R&D / Production

- Be an ally to individuals with rare diseases, focusing on innovation “only JCR can provide.”
-
JCR's mission as a biopharmaceutical company has been to engage in the R&D of therapeutic drugs for lysosomal storage diseases, a group of rare genetic diseases. Although drugs already exist for some of the many lysosomal storage diseases, they have not led to improvement in quality of life in practice, due to their limited efficacy and the significant side effects. In order to meet the demand for new treatment options by many patients and their families as soon as possible, we continue with our efforts in our R&D of new, high value-added biotherapeutics utilizing our vast repository of proprietary technologies and know-how.
-
-
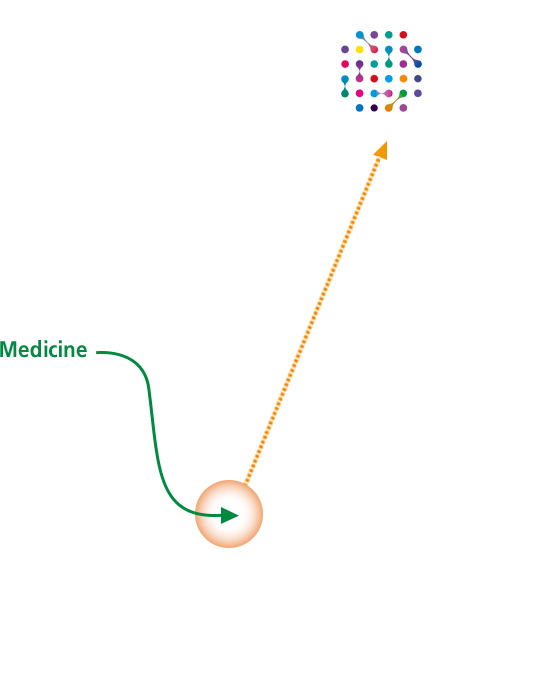
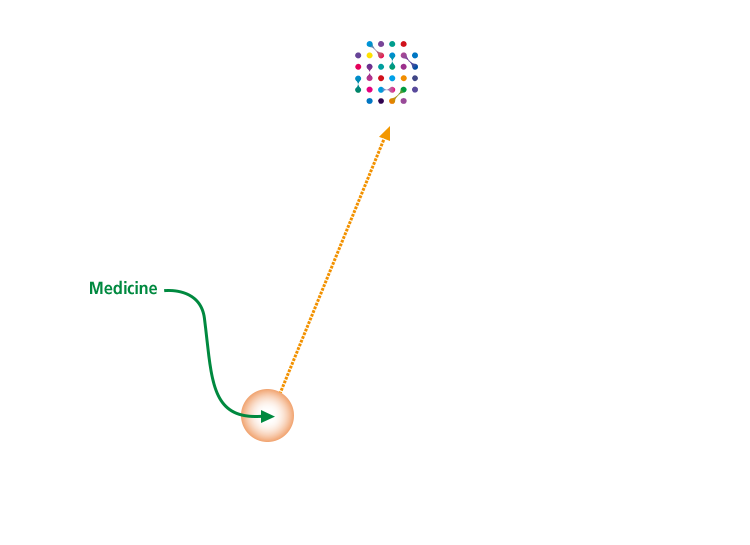
Blood-Brain Barrier Penetration Technology
"J-Brain Cargo®" -
The development of a high-level technology to deliver the active drug molecules to the central nervous system is required in order to improve the central nervous symptoms that induce developmental disorders, language disorders and such as seen in Hunter syndrome. JCR’s proprietary J-Brain Cargo® is the first technology proven to penetrate the Blood Brain Barrier to deliver drug to central nervous system in humans.
JCR has strived to create over J-Brain Cargo® technology applied drug candidates in the Lysosomal storage disorders area. -
-
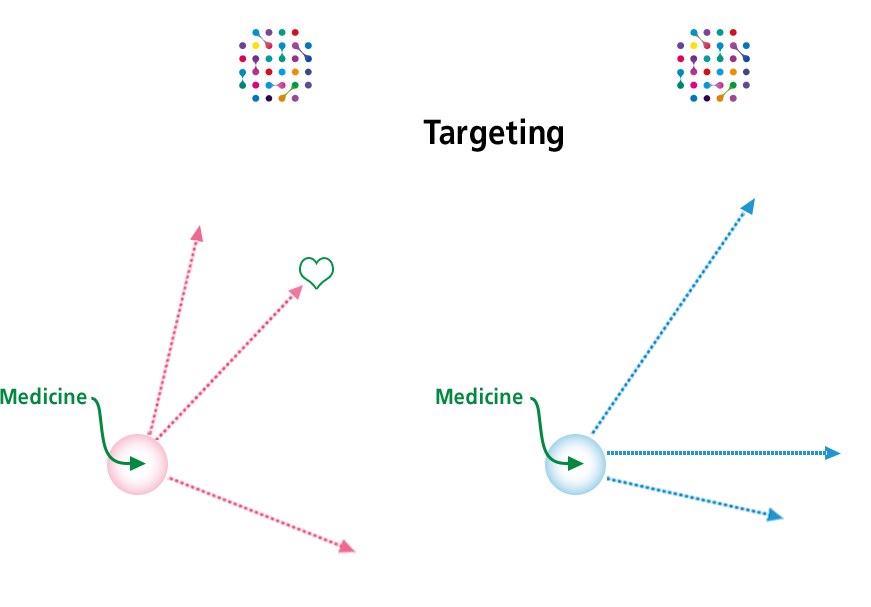
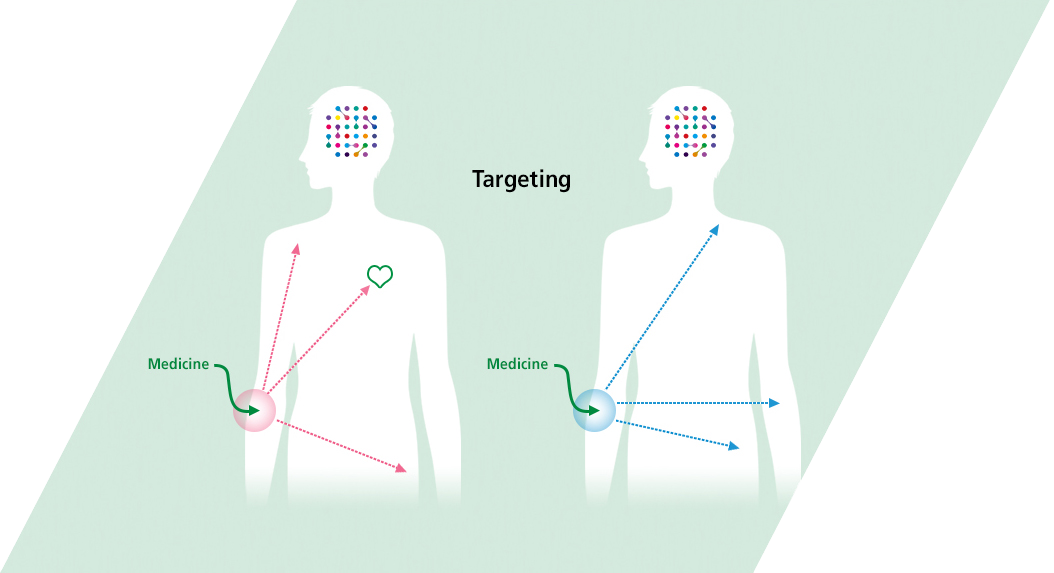
- Tissue- Targeting Technology
- J-Brain Cargo® is a technology that enables drug delivery not only across the blood-brain barrier, but also to targeted tissues throughout the body. For diseases other than LSDs, we will be able to target different diseases in the future.
Efforts Toward Development of Cell Therapy and Regenerative Medicine Technologies
-
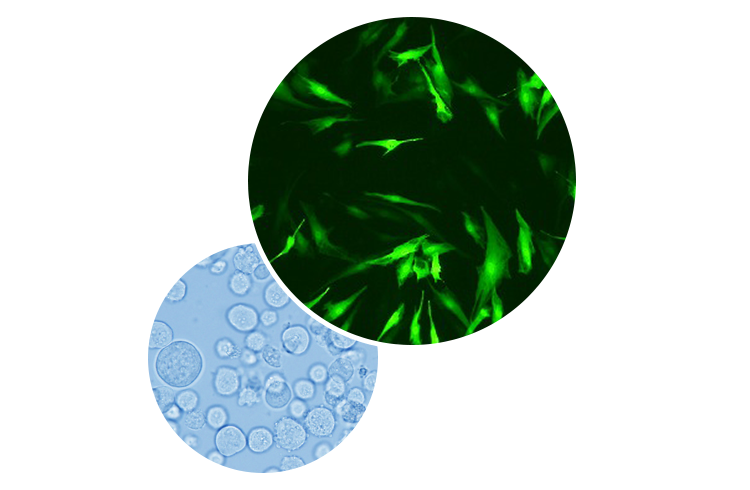
-
TEMCELL® HS Inj., the First
Allogeneic Regenerative Medicine in Japan - Human mesenchymal stem cells (MSCs) developed by JCR is produced from bone marrow aspirate collected from healthy donors. In the same way as ordinary drugs, these cells may be administered to any patients without donor-recipient HLA matching. Since MSC preparations can be produced and cryopreserved in advance, they show promise of becoming an innovative drug that is readily available, especially at times of emergencies. TEMCELL® obtained full regulatory approval on September 18, 2015 as the new treatment option for patients who are courageously fighting graft-versus-host disease (GVHD), a serious,life-threatening complication of allogeneic bone marrow transplant.
Distinguished Biopharmaceutical Manufacturing Facility in Japan
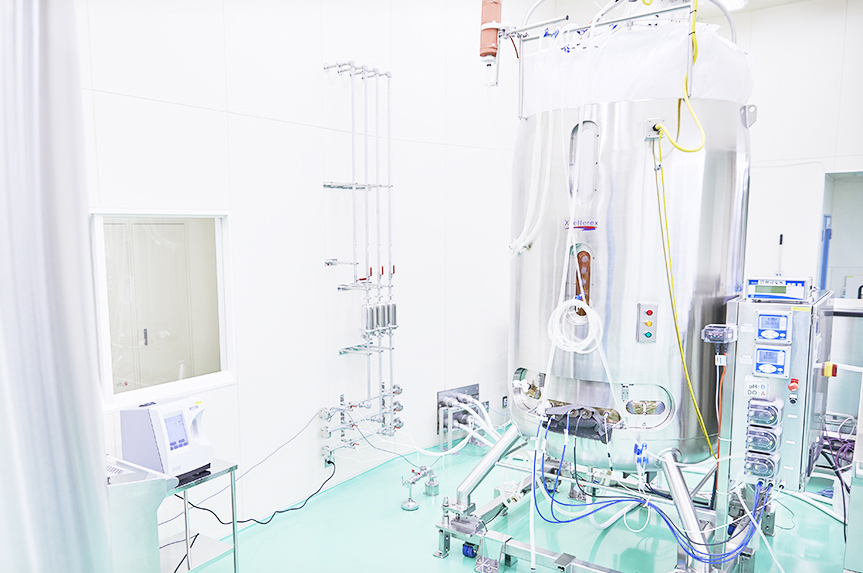
JCR has incorporated technologies that are one step ahead not only in R&D but also in production. In general, large-capacity bioreactors are required in the culturing process to manufacture biopharmaceuticals. However, although such bioreactors are efficient for large-scale production of a specific product, they are not suited for small-scale production of several products (e.g. orphan drugs) since they need to be washed with large amounts of distilled water after each culture batch. With the knowledge that we would start production of the orphan drugs currently being developed at JCR, we proceeded to construct an efficient system suitable for our needs by making use of the latest technologies such as the adaptation of bioreactors using disposable bags.

We will continue to make efforts to improve our technologies and gather information to provide a stable supply of high-quality, highly useful pharmaceuticals not only in Japan but also the world, and lead the Japanese biopharmaceutical industry now and in the future


 (229kb)
(229kb)